Question #35111
1 Answer
The answer is (a) high/low
Explanation:
Here's how you can think about this problem.
Lattice energy is defined as the energy given off when a crystal solid is formed from infinitely separated ions in the gaseous state.
Since this energy is given off, it naturally implies that it has a negative sign. However, you are actually interested in the opposite process, i.e. the energy needed to convert the crystal solid into infinitely separated ions in gaseous state.
When considered from this perspective, the lattice energy will have a positive sign.
The energy of solvation is actually the energy released when individual ions are surrounded in solution by the polar water molecules.
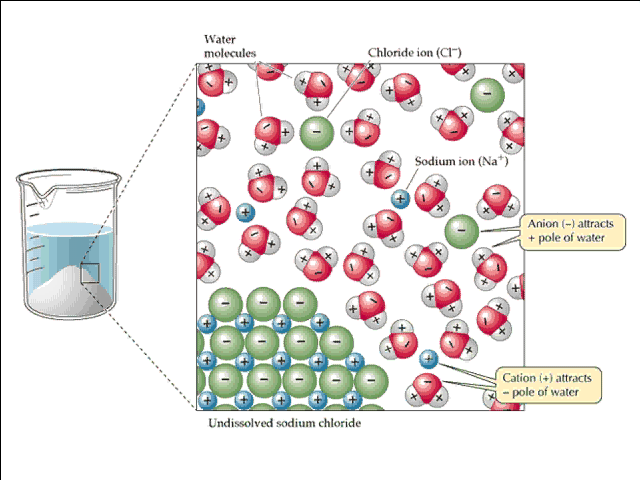
So the idea is that if the lattice energy is high and the solvation energy is low, then the energy needed to break up the ions from the crystal structure will exceed the energy released when the molecules of water bond to these ions.
In other words, if the energy needed to break up the ions, i.e. the energy cost, is greater than the energy released when solvation takes place, i.e. the energy gain, then the compound will most likely be insoluble.
As a rule of thumb, the lattice energy must be comparable to the energy of solvation if the compound is to dissolve.
In general, the solubility of ionic compounds will decrease with an increase in lattice energy.

