Question #65437
1 Answer
QUANTUM NUMBERS
The three main quantum numbers describe the shape, energy level, and orientation of the atomic orbitals.
There is a fourth (
#n# is the principal quantum number which describes the energy level.
#n = 1, 2, 3, . . . # and is always a positive integer.
#l# is the orbital angular momentum quantum number which describes the shape of the orbital.#l = 0, 1, 2, 3, . . . , n-1# .
#l = 0, 1, 2, 3, ...# for#s, p, d, f, ...# orbitals, respectively.
#m# (more specifically,#m_l# ) is the magnetic quantum number, which corresponds to each unique orbital orientation.#m_l# takes on the set of integers from#-l# to#l# , i.e.
#m_l = {0, pm1, pm2, . . . , pml}# .
#m_s# (see why we need to say#m_l# ?) is the electron spin quantum number, which describes the spin of the electron. It is only#pm1/2# , independent of the other quantum numbers.
2P ATOMIC ORBITAL
For example, a
#n = 2# since it's#color(highlight)(2)p# #l = 1# since it's#2color(highlight)(p)# #m_l = {0, pm1, . . . , pm l} = {-1, 0, +1}#
(if#l = 2# , then#m_l = {-2, -1, 0, +1, +2}# )
If the orbital has an electron, the electron's
We cannot say which electron is which, because they are indistinguishable.
The
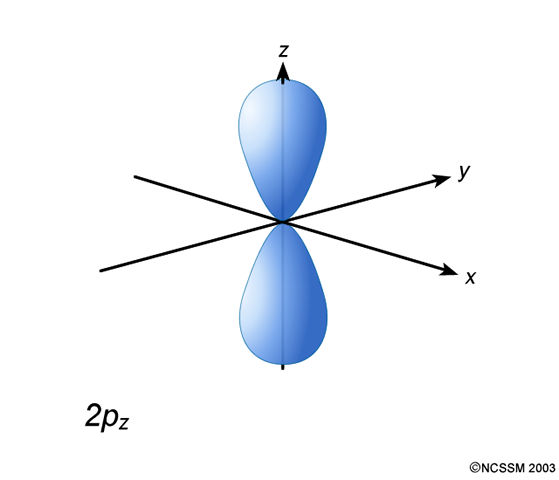
- While
#m_l = 0# , that corresponds to the#2p_z# orbital. - While
#m_l = -1# , that corresponds to the#2p_x# or#2p_y# orbital. - While
#m_l = +1# , that corresponds to the#2p_y# or#2p_x# orbital. (The uniqueness that#m_l = pm1# matters, but the choice of assigning either to#p_x# or#p_y# is arbitrary.)
Lastly, the observations you learn from General Chemistry describe the idea that electrons fill the orbitals:
- with the lowest energy first, in part due to
#n# (Aufbau Principle) - one at a time
- in such a way that
#m_s# is not the same for any two electrons both in a doubly-occupied orbital (Pauli Exclusion Principle).

