Can a strong nucleophile be a weak base?
1 Answer
Yes, a strong nucleophile can be a weak base.
Explanation:
In general, good bases are also good nucleophiles.
But weak bases can also be good nucleophiles. They fall into three classes.
i) Anions of large atoms
This means that they can start the bonding process while still some distance away from the substrate.
ii) Anions that are stabilized by resonance
The important members of this group are the carboxylate ions such as acetate ion (
iii) Neutral bases that have lone pairs on less electronegative atoms
These include amines (
Of course, these bases are not as nucleophilic as the strong bases, but they are still strong nucleophiles.
The chart below lists the nucleophilicity of some common bases.
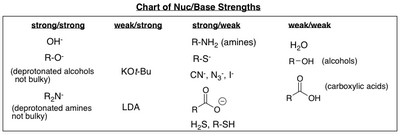
(from www.chem.sc.edu)

