How does an aromatic hydrocarbon differ from a cycloalkane?
1 Answer
There is a huge difference.
Explanation:
Aromatic carbons have π bonds (Double Bonds) whereas Cycloalkanes don't have π bonds.
Aromatic Hydrocarbon consists of typically a Benzene ring or multiple Benzene rings bonded together, which means that it is 6 Carbons bonded in a cyclic (ring) structure with alternating double bonds. I.e single bond, double bond, single bond etc. To clarify here is a picture of Benzene:
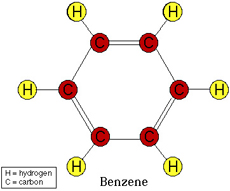
Image from: 
Cycloalkanes on the other hand as depicted in the name "alkane" consist of a cyclic structure with n (n = 1,2,3,4...) number of Carbon atoms but only have single bonds between the Carbons.
This results in many differences in chemical reactivity of both types of compounds: Aromatics give electrophillic substitution reactions where as cycloalkane gives addition , substitution and many more reactions.


