Question #b6eea
1 Answer
Here's how you can think about what's going on.
Explanation:
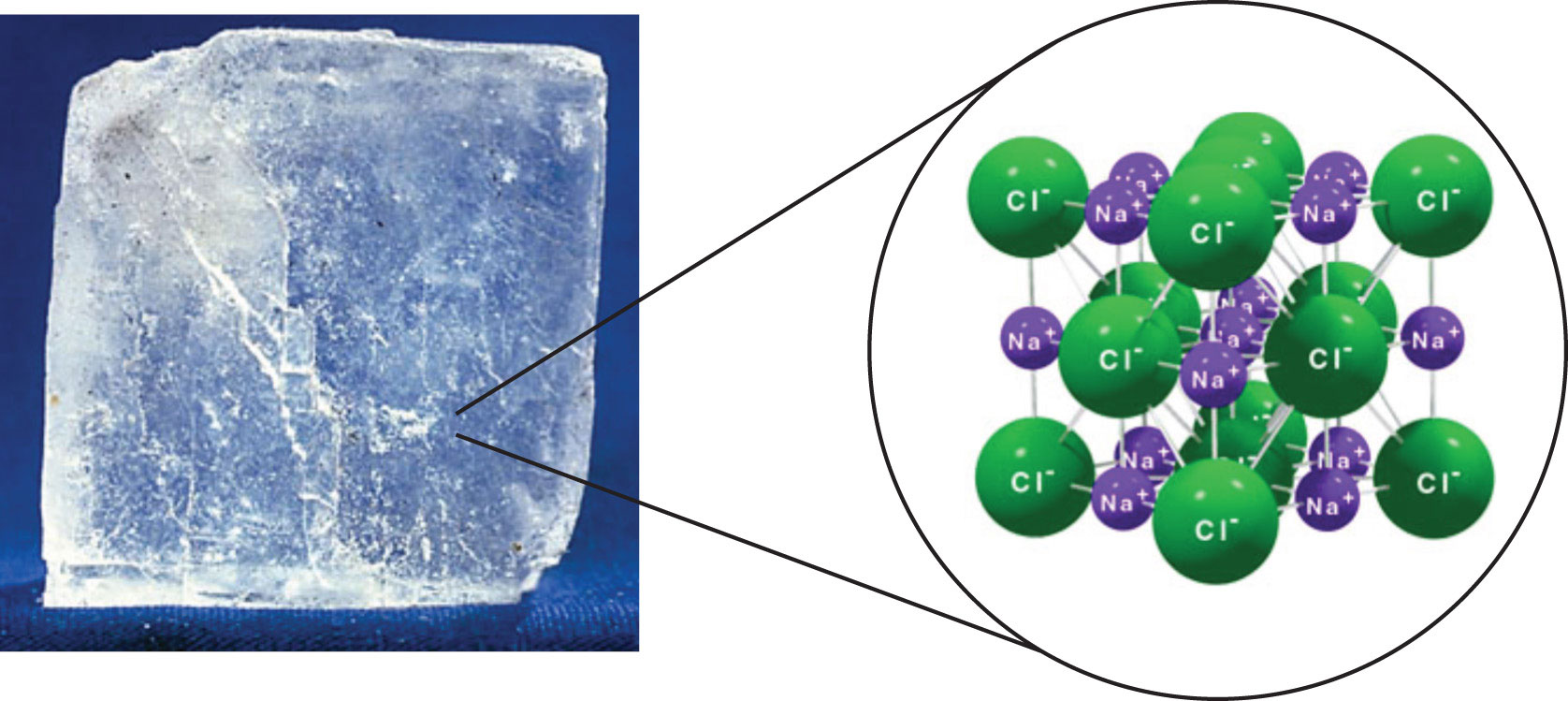
Sodium chloride,
These ions are arranged in a vast crystal lattice structure, with sodium cation being surrounded by six chloride anions and each chloride anion being surrounded by six sodium cations.
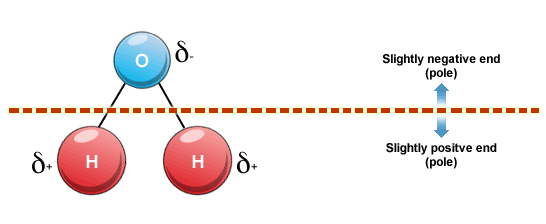
As you know, water is a polar solvent, which is another way of saying that each individual water molecule is polar.
More specifically, each water molecule has partial positive charges located on its hydrogen atoms and a partial negative charge located on its oxygen atom.
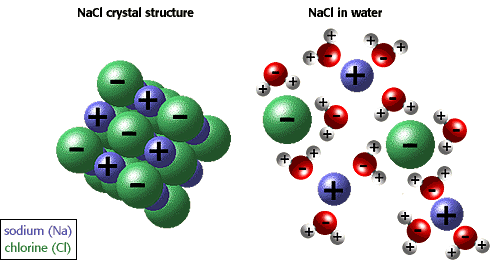
Now, when you place a sodium chloride crystal in water, an electrostatic attraction will exist between the sodium cations, which are positively charged, and the oxygen end of the neighbouring water molecules.
Likewise, the chloride anions, which are negatively charged, will be attracted to the hydrogen end of the neighbouring water molecules.
In sodium chloride's case, the attraction between the ions and the water molecules is greater than the attraction between the ions that make up the crystal structure.
This means that the water molecules will be able to break the crystal structure of the solid by taking away the sodium and chloride ions.
Each sodium cation will be surrounded by water molecules that have the hydrogen atoms pointed out and each chloride anion will be surrounded by water molecules that have the oxygen atoms pointed out.
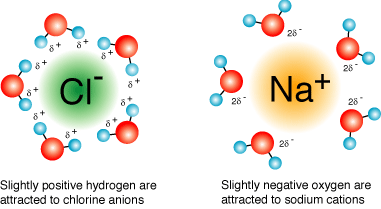
These water molecules form a solvation shell around each ion, essentially cancelling any electrostatic attraction that could exist between the sodium cations and chloride anions.
As a result, the sodium chloride will no longer exist as sodium chloride in aqueous soltuion, it will exist as individual ions.
#"NaCl"_((s)) stackrel(color(red)(aq))(->) "Na"_text(aq])^(+) + "Cl"_text((aq])^(-)#

