A reaction starting with 8.0 g of benzoic acid, excess methanol, and excess phenyl magnesium bromide.What would be the expected theoretical yield of triphylmethanol?? If 5.36 g of triphenylmethanol is end product, what is % yield?Show all calculation.
1 Answer
This all belongs to the same lab report you are probably doing right now. See this for the mechanism after you form methyl benzoate:
The only difference here is that now you are forming methyl benzoate using benzoic acid and excess methanol. Excess because this reaction is slow.
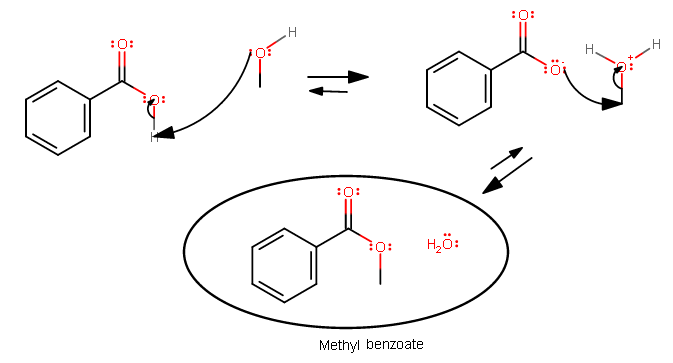
You know that benzoic acid must be the limiting reagent in the entire reaction from start to finish because everything else is in excess. You're really just driving the equilibrium forward.
You really only need one equivalent of methanol to react for this to work, and you only get one equivalent of methyl benzoate if this works.
When you react with excess phenyl magnesium bromide with this, you need excess of that due to some steric hindrance of having three benzene rings on one product. You started with one ring and you get two, so you get two equivalents of phenyl magnesium bromide that need to react.
Dehydration of the carboxylic acid into an ester:
Dry over
Grignard reaction to form a tertiary aromatic alcohol:
Our focus is the main product, triphenylmethanol. You only need one equivalent of benzoic acid combined with two equivalents of phenyl magnesium bromide, so we're good to go now.
Therefore:
To be honest, that's a pretty normal yield to get. (On the final lab you may do this year, don't be surprised if you only get
If you are still unsure, feel free to ask your professor to confirm.

