Write the overall reaction for reaction of methyl benzoate with excess phenyl magnesium brimide in ether, followed by H3O+. ????
1 Answer
Sep 6, 2015
The mechanism for this is something like this:
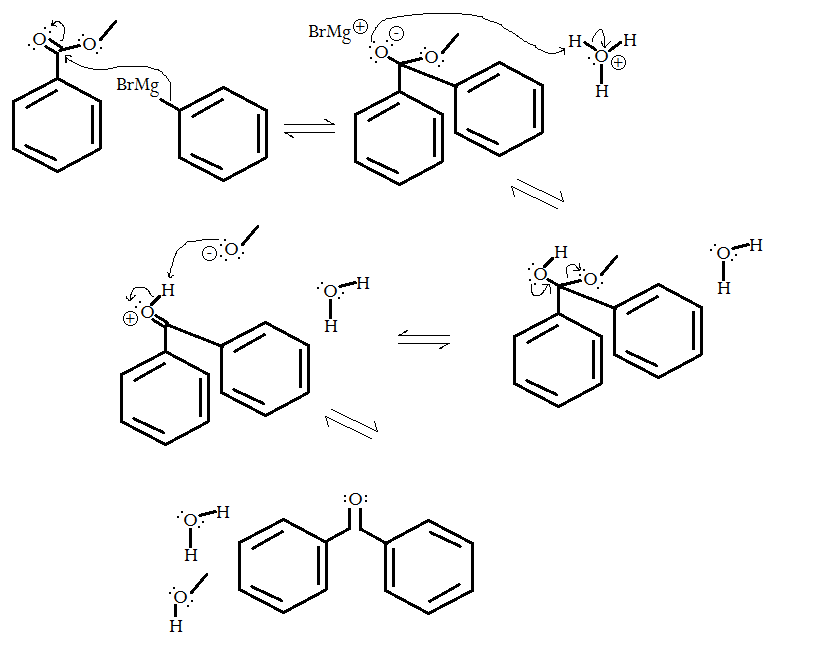
- The magnesium bromide acts as a Lewis acid, turning the phenyl group into a nucleophile. Naturally, it backside-attacks the most susceptible electrophilic center, forming a fairly sterically-hindered tetrahedral intermediate. It is excess reactant so that it happens to a reasonable yield.
- The negatively-charged oxygen grabs a proton from the acid that you add afterwards.
- Electron conjugation from the newly-formed hydroxyl group's oxygen forces the methoxy leaving group to break off, a rapid intramolecular process. The pKa of methanol is about
#15.5# , but the pKa of benzene is about#43# , so there is pretty much no chance (#1# in#10^27.5)# of the phenyl group breaking back off. - The reaction finishes when the methanolate grabs the proton off of the protonated final product. The methanolate does so rather than the water (
#10^6.1# times as often), since the pKa of methanol (#~15.5# ) is greater than the pKa of hydronium (#~9.4# ), and acid-base equilibrium lies on the side of the weaker acid or stronger base. - Repeat the curved arrows and electron flow in steps 1 and 2 with another equivalent of phenyl magnesium bromide.
Your final product is triphenylmethanol.

