When does electron capture occur instead of positron emission?
1 Answer
Electron capture occurs when the loss in mass is less than that of two electrons.
Explanation:
Nuclear stability depends on the neutron:proton ratio.
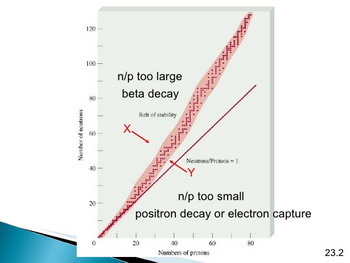
(from www.slideshare.net)
Nuclei that have a neutron: proton ratio that is too low can become stable by positron emission or by electron capture.
1. Positron emission
In positron emission, a proton is converted to a neutron by emitting a positron and a neutrino.
For example,
Positron emission occurs spontaneously when the mass of the parent atom is greater than the mass of the daughter atom plus two electrons:
2. Electron capture
In electron capture, an outside electron is pulled inside the nucleus and combined with a proton to make a neutron, emitting only a neutrino.
For example,
Electron capture happens most often in the heavier neutron-deficient elements where the mass change is smaller than that from positron emission.
When the loss in mass is less than that of two electrons, positron emission cannot occur, but electron capture will still be spontaneous.

