Question #f7e3f
1 Answer
No reaction takes place.
Explanation:
You're actually dealing with tert-butanol, a tertiary alcohol, which cannot be oxidized by acidified potassium dichromate.
Acidified potassium dichromate is actually a solution that contains sulfuric acid and potassium dicromate. This solution is used to oxidize primary and secondary alcohols.
Primary alcohols can either be partially oxidized to aldehydes, or completely oxidized to carboxilic acids.
Likewise, secondary alcohols can be oxidized to ketones.
Now, the idea here is that the structure of the tertiary alcohol does not allow for their oxidation because of the fact that the carbon atom that has the hydroxyl group,
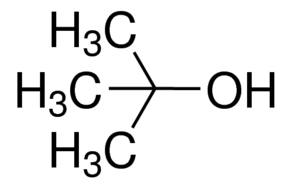
The key for the oxidation of primary and secondary alcohols is that the carbon to which the hydroxyl group is attached can form a double bond with the oxygen atom after a hydrogen atom that it's attached to gets plucked away.
This hydrogen atom and the hydrogen atom attached to the oxygen atom will then form a water molecule, allowing for the double bond between carbon and oxygen to form.
In the case of tertiary alcohols, that cannot happen because you do not have a hydrogen atom attached to that carbon.
This is why tertiary alcohols are said to be resistant to oxidation.

