Why is the #pi# bond between the two central carbon atoms destroyed halfway through the rotation from cis- to trans-2-butene?
1 Answer
Oct 12, 2015
Because it's not a rotation of the double bond itself. A cis and trans molecule are geometric isomers, not conformational isomers.
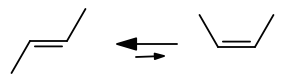
The bond is too rigid to rotate, so the
For example, azo dyes are sensitive to light and experience this effect of trans-cis isomerism, called photoisomerism, with times on the order of
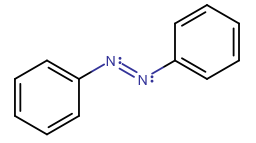
https://en.wikipedia.org/wiki/Azobenzene#Trans-cis_isomerization

