Which complex ion geometry has the potential to exhibit cis– trans isomerism: linear, tetrahedral, square planar, octahedral?
1 Answer
Oct 23, 2015
Only the square planar and octahedral complexes can exhibit cis-trans isomerism.
Explanation:
Linear and tetrahedral complexes cannot exhibit cis-trans isomerism, but square planar and octahedral complexes can.
Square planar complexes
In the two complexes shown below, one has the two iodine atoms adjacent to each other (cis), and the other has the iodine atoms across from each other (trans)
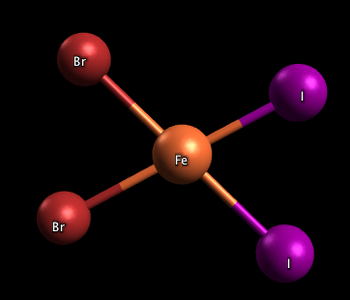
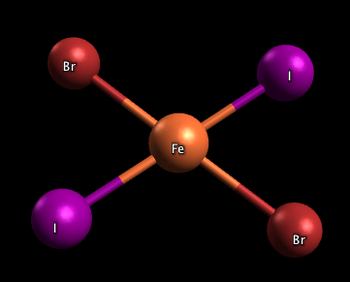
(from chemwiki.ucdavis.edu)
Octahedral complex ions*
The octahedral tetraamminedichloridocobalt(III) ion,
(from wps.prenhall.com)
The cis isomer has the chlorine atoms (green balls) adjacent to each other, and the trans isomer has them across from each other.



