Which has the larger atomic radius: Cl- or K+?
1 Answer
Explanation:
Atomic size is linked to two important concepts
- the energy level on which the outermost electrons reside
- the effective nuclear charge of the nucleus
The first concepts is useful when dealing with atoms that are located in different periods of the periodic table.
In this case, the higher in energy an atom's outermost electrons are, the further away from the nucleus they will be. This in turn implies a larger atomic radius.
In the case of a neutral potassium atom, you know that its electron configuration looks like this
"K: " 1s^2 2s^2 2p^6 3s^2 3p^6 color(red)(4)s^1
Potassium has its outermost electron on the fourth energy level. When the potassium cation is formed, that outermost electron is lost, which means that the electron configuration of
"K"^(+): " 1s^2 2s^2 2p^6 color(red)(3)s^2 color(red)(3)p^6
Its outermost electrons are now located on the third energy level.
Now take a look at a neutral chlorine atom
"Cl: " 1s^2 2s^2 2p^6 color(red)(3)s^2 color(red)(3)p^5
Its outermost electrons are alredy on the third energy level. When the chloride anion is formed, an electron is being added to that same energy level, so that you have
"Cl"^(-): " 1s^2 2s^2 2p^6 color(red)(3)s^2 color(red)(3)p^6
The two ions have the same electron configuration, which means that they are isoelectronic.
Now turn to the second concept, effective nuclear charge.
Both ions have their outermost electrons located on the same energy level, but they do not have the same ionic radius.
That happens because the number of protons each species has in its nucleus varies.
More specifically, potassium, which has an atomic number equal to
This is important because in the case of the potassium atom, the outermost electrons will be attracted by the nucleus more.
In other words,
This will compress the energy levels a bit and make the ionic radius smaller for the potassium cation.
Therefore, the chloride anion will have the larger atomic radius.
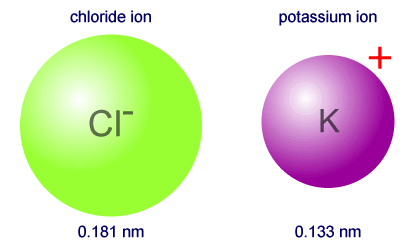 http://www.ibchem.com/IB16/05.22.htm
http://www.ibchem.com/IB16/05.22.htm

