When balancing a chemical equation, why can you change coefficients, but not subscripts?
2 Answers
When you change the coefficients, you're only changing the number of molecules of that particular substance. However, when you change the subscripts, you are changing the substance itself, which will make your chemical equation wrong.
because changing subscripts would mean you are changing the basic composition of the substance.
Explanation:
Let's take a look at the simplest example:
The equation above means that in order to "create" 2 molecules of water (
Now take a look at the illustration. This is what happens when you change the subscript in balancing equations instead of just putting coefficients.
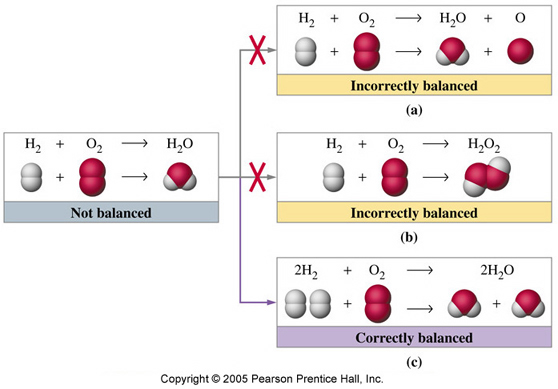
As you can see, balancing the equation by changing the subscripts basically means that you are making a "new" substance rather than the one originally given in the equation.


