What is a limiting reactant?
2 Answers
Limiting reactant is the reactant that limits the amount of a product that can be formed in a chemical reaction.
Explanation:
For example, suppose we have 4 bolts and 8 nuts. No matter how many nuts are there, we need only 4 nuts as we have got 4 bolts. Same thing about a chemical reaction.
If the limiting reactant is fully consumed, the reaction will stop even if the other reactant still remains unreacted. That reactant is called excess reactant. I hope this picture will make it easier for you to understand.
< Sorry I couldn't find a nut-bolt one, but this car body and tire example will do>
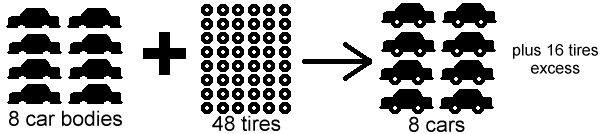 https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
The limiting reactant in a chemical equation is the reactant that is completely used up at the end of the reaction.
Explanation:
I would watch this video from Khan Academy about limiting reactants if you want some more info!



