What are the quantum numbers necessary to specify a #5p# subshell?
1 Answer
Explanation:
As you know, quantum numbers are sused to describe the exact location and spin an electron can have in an atom.
A total of
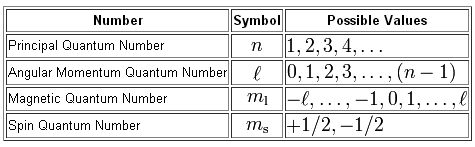
Now, the principal equantum number,
Each energy level contains a specific number of subshells, given the possible values the angular momentum quantum number,
For example, the second energy level is characterized by
This means that the second energy level will have a total of
In your case, the energy level is given by the number that's placed in front of the letter p, which denotes a specific subshell.
So, the principal quantum number,
Now, the any p-subshell is characterized by
Therefore, the value of angula momentum quantum number will be

