What bonding feature allows you to differentiate among an alkane, alkene, alkyne, and an aromatic?
1 Answer
Nov 8, 2015
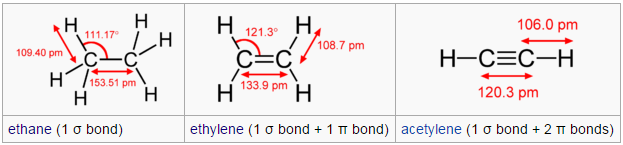
The number of electrons involved in the bonding. Also bonds angles and distances between atoms.
Explanation:
The basic difference from this groups is the number of electrons involved in the bonding. 2 electrons for an alkene (single bond), 4 electrons for an alkene (double bond), 6 electrons for an alkyne (triple bond), and for aromatic group it's a double bond too but in this case the electrons are in 'resonance'.
The number of electrons involved affects the strength of the bond so a triple bond is stronger than a double bond, and a double is stronger than a single bond. So if the bond is stronger the atoms are closer, and the bond angle is wider.