Question #a8fdc
1 Answer
Nov 11, 2015
Covalent.
Explanation:
The bond between the hydrogen atom
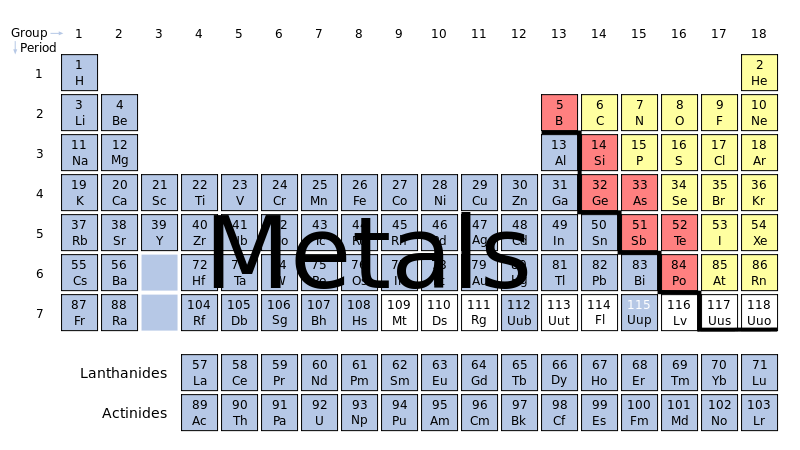
A simple way to know whether the bond is ionic or covalent, is to check whether one of the elements is a metal or not.
Ionic bond forms between one metal element and one non metal. Example:
Covalent bond forms between two non metal elements . Example,
Metal elements are located to the left of the stairs in the periodic table. (See below image).