What are the possible quantum numbers for the 6d orbital?
1 Answer
Here's what I got.
Explanation:
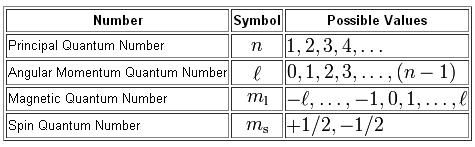
In order for an electron to reside in the d-subshell of any energy level, starting with the third one, it must have a distinct value for its angular momentum quantum number,
More specifically, the only value of
In the case of the 6d-subshell, the principal quantum number ,
Now, any d-subshell will contain a total of
For a d-subshell,
#m_l = {-2; -1; 0; 1; 2}#
Now, the spin quantum number,
#m_s = -1/2 -># describes an electron with spin-down
#m_s = +1/2 -># describes an electron with spin-up
So, to sum this up, the electrons located in the 6d-subshell will have
#n = 6 -># the sixth energy level#l=2 -># the d-subshell#m_l = { -2; -1; 0; 1; 2} -># the five d-orbitals found in the d-subshell#m_s = +- 1/2 -># either spin-up or spin-down

