What is the structure of the acetal formed by reaction of ethanol with acetophenone?
1 Answer
Nov 17, 2015
The structure of the acetal is
Explanation:
The structure of the acetal is
Ketones react in the presence of acids to form hemiacetals, and the hemiacetals can react further to form acetals.
The general equation is
The mechanism is
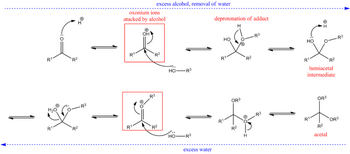
(from en.wikipedia.org)
In the addition of ethanol to acetophenone,
So the structure of the acetal is given by the equation

