What do you get when you react benzaldehyde and acetophenone with a base?
1 Answer
Nov 20, 2015
I'm assuming with heat involved?
You would get the product of an aldol condensation. Specifically for your case, a chalcone.
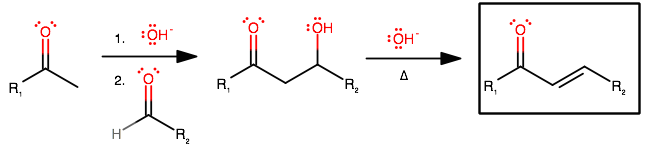
The mechanism is:
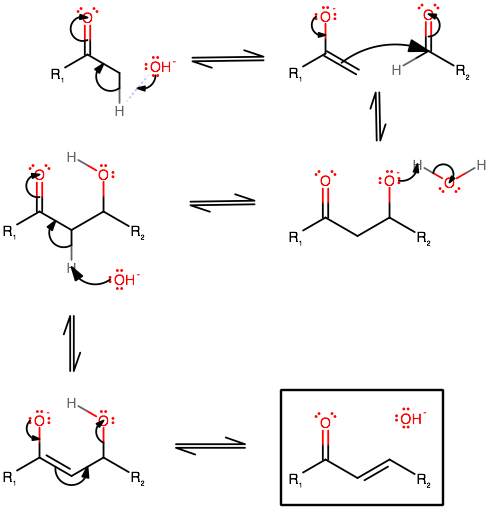
Can you analogize and draw the mechanism for your compounds? This seems like a question related to a lab, and you should be able to do it from here.
CHALLENGE: Do you know why, conformationally, the second half of the reaction does not involve

