What are the products of the reaction between aqueous solutions of silver nitrate and calcium bromide?
1 Answer
Here's what I got.
Explanation:
In order to be able to answer this question, you need to be familiar with the Solubility Rules for ionic compounds in aqueous solution
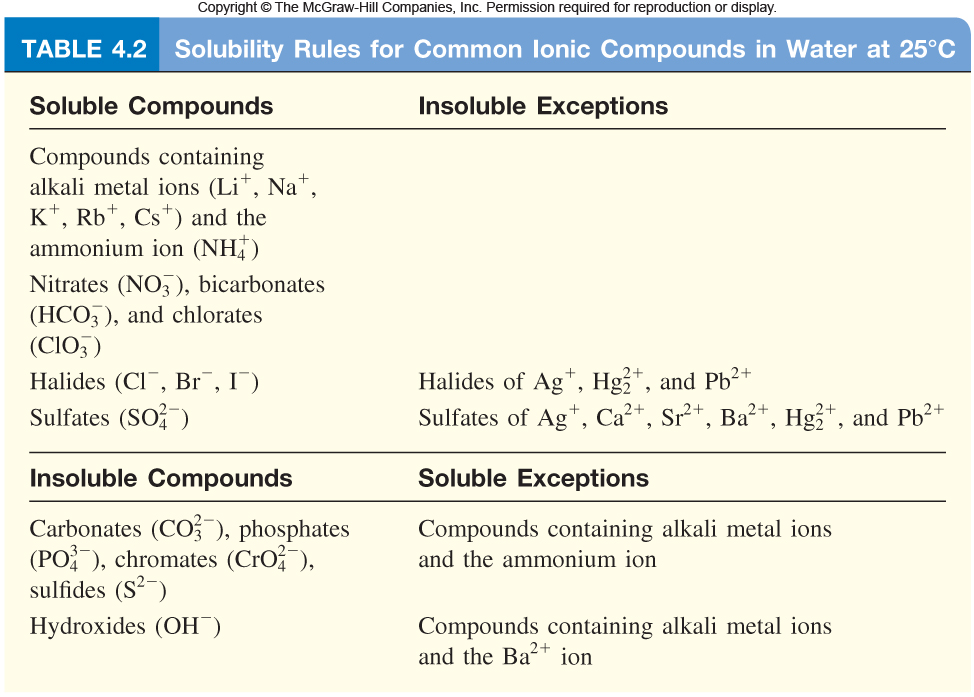
So, you're mixing two solutions, one containing silver nitrate,
Both these ionic compounds a resoluble in aqueous solution, which means that they will exist as cations and anions. More specifically, the first solution will contain silver cations,
#"AgNO"_text(3(aq]) -> "Ag"_text((aq])^(+) + "NO"_text(3(aq])^(-)#
The second solution will contain calcium cations,
#"CaBr"_text(2(aq]) -> "Ca"_text((aq])^(2+) + 2"Br"_text((aq])^(-)#
So what's going to happen when you mix these solutions? Take a look at the Solubility Rules.
Notice that nitrates, which are ionic compounds that contain the nitrate anion, are soluble in aqueous solution without exception.
On the other hand, bromides, which are ionic compounds that contain the bromide anion, are soluble with three exceptions, including with silver cations,
This means that the silver cations will bond to the bromide anions and form an insoluble solid that precipitates out of solution.
The complete ionic equation will look like this
#2"Ag"_text((aq])^(+) + 2"NO"_text(3(aq])^(-) + "Ca"_text((aq])^(2+) + 2"Br"_text((aq])^(-) -> 2"AgBr"_text((s]) darr + "Ca"_text((aq])^(2+) + 2"NO"_text(3(aq])^(-)#
The net ionic equation, which doesn't show spectator ions, will look like this - do not forget to balance the cations and anions!
#2"Ag"_text((aq])^(+) + 2"Br"_text((aq])^(-) -> 2"AgBr"_text((s]) darr#
which is actually
#"Ag"_text((aq])^(+) + "Br"_text((aq])^(-) -> "AgBr"_text((s]) darr#
So, this double replacement reaction will produce silver bromide,

