Question #a0bc7
1 Answer
Explanation:
As you know, plants use the photosynthesis reaction to convert carbon dioxide and water, in the presence of light and chlorophyll, into glucose and oxygen gas.
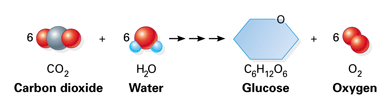
The key to this problem is the balanced chemical equation for the photosynthesis reaction, which looks like this
#6"CO"_text(2(g]) + 6"H"_2"O"_text((l]) + color(blue)("light") -> "C"_6"H"_12"O"_text(6(s]) + 6"O"_text(2(g])#
Notice that you have a
More specifically, for every
In your case, you want the chamber to maintain an initial number of
#0.001 color(red)(cancel(color(black)("moles O"_2))) * "6 moles CO"_2/(6color(red)(cancel(color(black)("moles O"_2)))) = "0.001 moles CO"_2#
This means that the number of moles of carbon dioxide will decrease by

