What is the difference between a limiting reactant and an excess reactant?
1 Answer
Nov 30, 2015
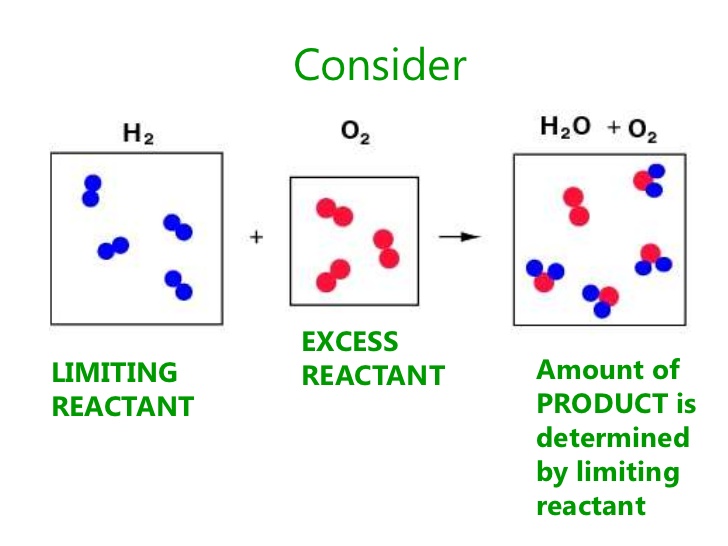
A limiting reactant is one in which it produces the least amount of product. An excess reactant is one in which it produces more of a product than the limiting reactant.
Explanation:
The excess reactant is present in the products because it did not completely react because there was not enough of the limiting reactant. The limiting reactant determines how much product can be produced.
The image below represents the reaction between hydrogen and oxygen gas to produce water. 4 molecules of