Why is benzene not considered a cycloalkene?
1 Answer
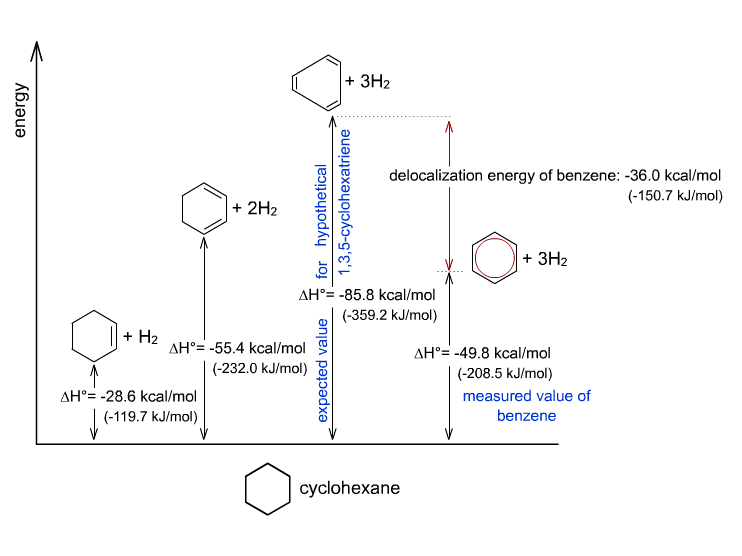
Because if it truly was, its enthalpy of hydrogenation (the enthalpy due to a reaction in which a substance is reduced by adding hydrogen) would be very similar to that of the theoretical, localized 1,3,5-cyclohexatriene.
#DeltaH_(rxn, "1,3,5-cyclohexatriene")^@ ~~ "-359.2 kJ/mol"#
#DeltaH_(rxn, "Benzene")^@ ~~ "-208.5 kJ/mol"#
It's clear that reducing benzene releases less energy, which means that its
As a result, an additional stabilization effect (due to resonance delocalization of the
You can see that in the diagram here:
To reiterate, the left resonance structure here as-drawn is not the same as the right resonance hybrid structure here: