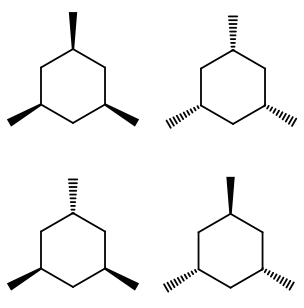
How many stereoisomers and chiral carbons are there on a 1,3,5-trimethyl cyclohexane molecule?
1 Answer
Dec 4, 2015
Well, there aren't really any chiral carbons here, because each supposed chiral carbon only has three unique substituents (methyl, hydride, methylene), not four unique ones. The molecule as a whole isn't really chiral either, since its mirror image is superposable onto the original.
However, you can still align each methyl in the rear or the front, and that does shift the chair flip equilibrium of the molecule by affecting its stability; hence, it does make it a different, unique configuration of the molecule.
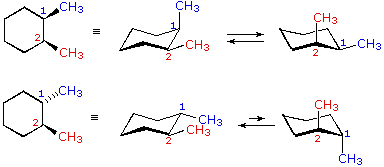
Due to symmetry, you can have all methyls in the rear/front, one in the rear/front and two in the front/rear, or two in the rear/front and one in the front/rear.
That makes four stereoisomers, but no true chiral carbons.