Lactic acid HC3H5O3 has one acidic hydrogen. A 0.10 M solution of lactic acid has the concentration of hydronium ion of 0.00363 M. Calculate Ka for lactic acid?
1 Answer
Explanation:
All you have to do in order to find the value of the acid dissociation constant,
Being a weak acid, lactic acid will not dissociate completely to form lactate,
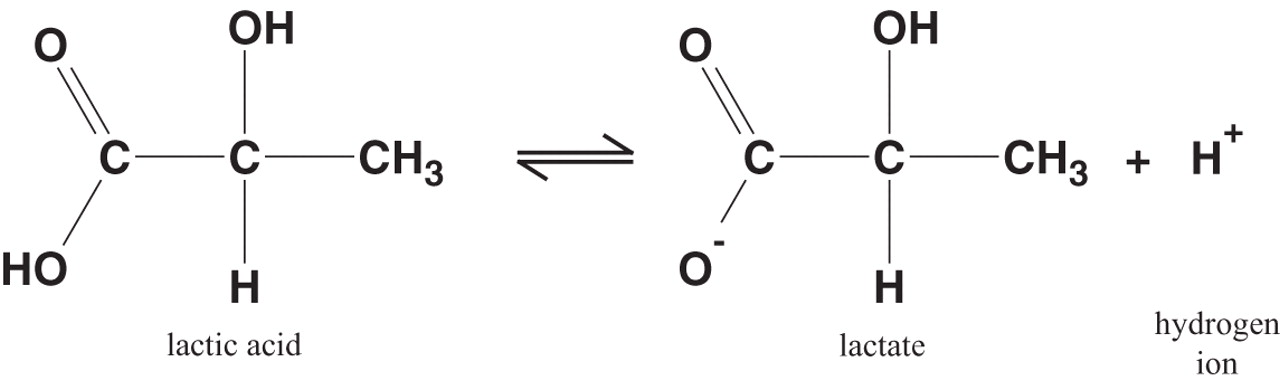
Use an ICE table to help you find the equilibrium concentration of the lactic acid
#" ""HC"_3"H"_5"O"_text(3(aq]) + "H"_2"O"_text((l]) rightleftharpoons "C"_3"H"_5"O"_text(3(aq])^(-) + "H"_3"O"_text((aq])^(+)#
By definition, the acid dissociation constant will be equal to
#K_a = ( ["C"_3"H"_5"O"_3^(-)] * ["H"_3"O"^(+)])/(["HC"_3"H"_5"O"_3])#
You know that the equilibrium concentration of hydronium ions is
#["H"_3"O"^(+)] = x = "0.00363 M"#
This means that the equilibrium concentration of the lactic acid will be
#["HC"_3"H"_5"O"_3] = 0.10 - x#
#["HC"_3"H"_5"O"_3] = "0.10 M" - "0.00363 M" = "0.09637 M"#
This means that the value of the acid dissociation constant will be
#K_a = ( 0.00363 * 0.00363)/(0.09637) = 1.367 * 10^(-4)#
Rounded to two sig figs, the number of sig figs you have for the molarity of the lactic acid solution, the answer will be
#K_a = color(green)(1.4 * 10^(-4))#

