The density of balsa wood is about #170# #kg##/##m^3#. How do you convert it to #g##/##cm^3#?
1 Answer
Explanation:
You need to use two conversion factors, one to take you from kilograms to grams and one to take you from cubic meters to cubic centimeters.
So, grab a metric prefix table and break down the two conversion factors
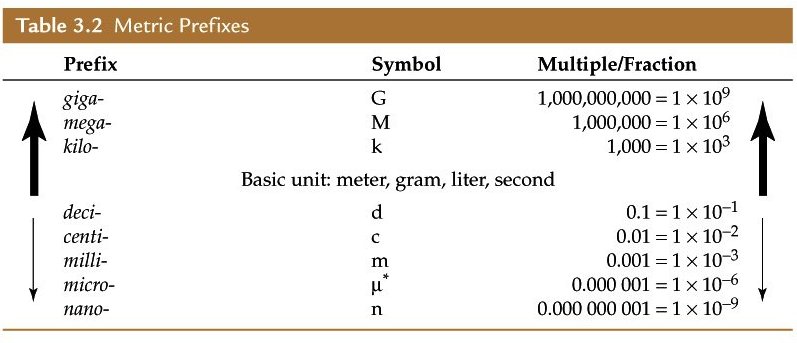
Start by focusing on the mass. As you can see,
Let's convert kilograms per cubic meter to grams per cubic meter
#170 color(red)(cancel(color(black)("kg")))/"m"^3 * (10^3"g")/(1color(red)(cancel(color(black)("kg")))) = 170 * 10^3"g/m"^3#
Now focus on the volume. First, you know that
#"1 m"^3 = "1 m" xx "1 m" xx "1 m"#
By the same logic,
#"1 cm"^3 = "1 cm" xx "1 cm" xx "1 cm"#
As you an see in the table,
#"1 cm" = 1/10^2 "m" = 10^(-2)"m"#
Therefore,
#"1 cm"^3 = 10^(-2)"m" xx 10^(-2)"m" xx 10^(-2)"m" = 10^(-6)"m"^3#
Finally, you can convert the density from grams per cubic meter to grams per cubic centimeter
#170 * 10^3"g"/color(red)(cancel(color(black)("m"^3))) * (10^(-6)color(red)(cancel(color(black)("m"^3))))/("1 cm"^3) = color(green)("0.17 g/cm"^3)#

