What are the bond angles in the central atom of the following: #"NSF"#, #"OF"_2#, and #"IBr"_2^−"#?
1 Answer
The bond angles are slightly less than 120°, slightly less than 109.5 °, and 180 °, respectively.
Explanation:
The structure of
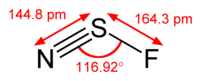
There is a lone pair on the
This is an
The theoretical bond angle is 120 °, but repulsion by the lone pairs decreases the bond angle to about 117 °.
The Lewis structure of

This is an
The theoretical bond angle is 109.5 °, but repulsions by the lone pairs decrease the bond angle to about 103 °.

The Lewis structure of
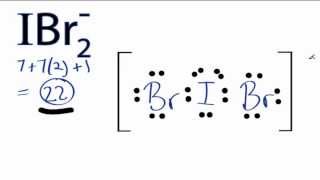
This is an

The lone pairs occupy the equatorial positions, with the
The bond angle is 180 °.

