How many stereoisomers are possible for CHBrClF provided that the central carbon has a square planar geometry?
1 Answer
Dec 24, 2015
There are three possible stereoisomers for a square planar
Explanation:
They are
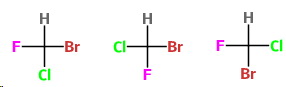
Start with
Then you can put
For the third isomer, put
There are no optical isomers for square planar

