Question #05209
1 Answer
The answer is (b)
Explanation:
Hydrochloric acid,
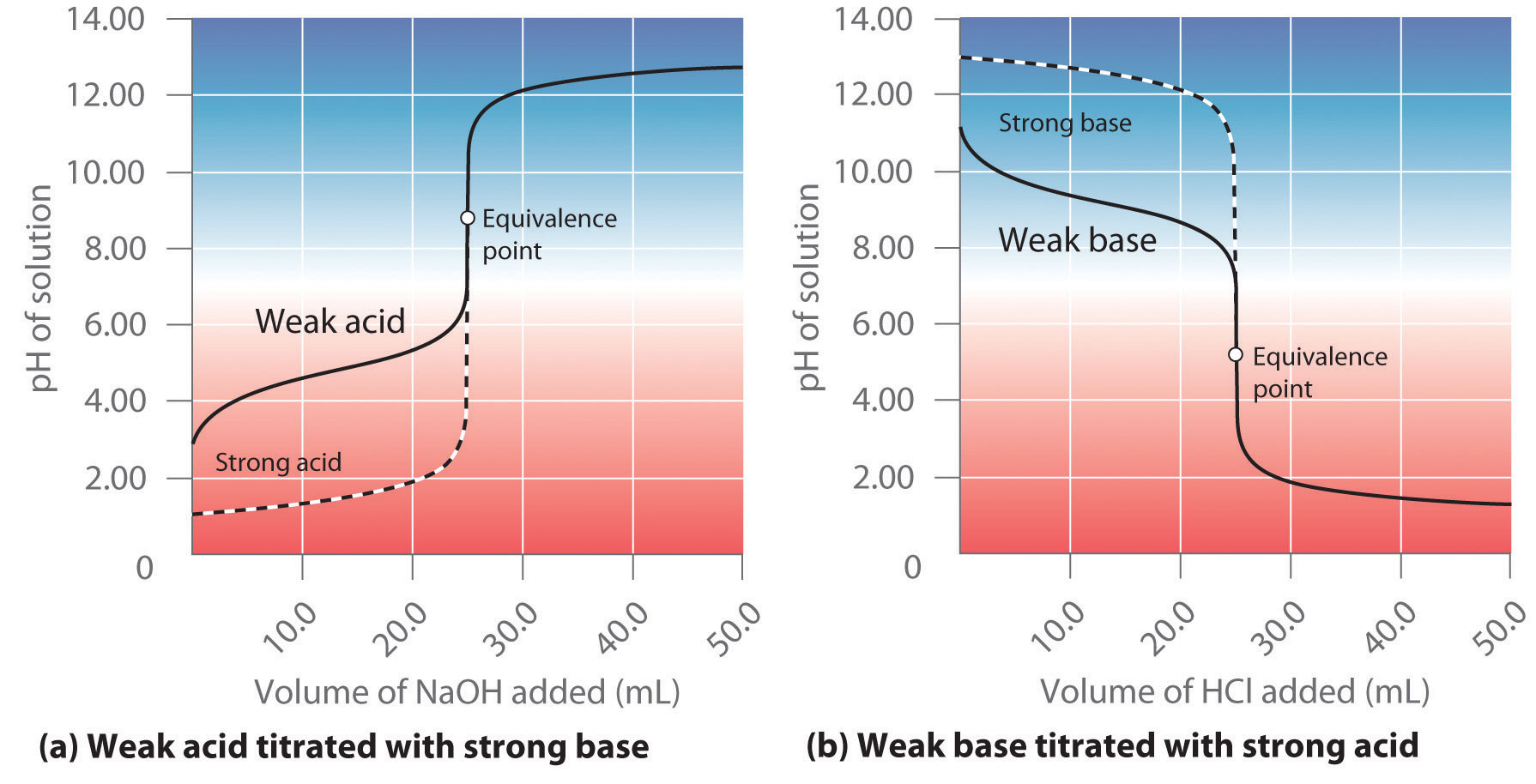
At equivalent point, both the strong acid and the weak base have been consumed by this neutralization reaction.
#"HCl"_text((aq]) + "NH"_text(3(aq]) -> "NH"_text(4(aq])^(+) + "Cl"_text((aq])^(-)#
The important thing to realize now is that the ammonium ion, which is the conjugate acid of ammonia, will actually react with the water molecules to reform some of the weak base and produce hydronium ions,
#"NH"_text(4(aq])^(+) + "H"_2"O"_text((aql]) rightleftharpoons "NH"_text(3(aq]) + "H"_3"O"_text((aq])^(+)#
Since the concentration of hydronium ions increases as a result of this equilibrium reaction, the pH of the solution will decrease.
This means that the titration of a strong acid with a weak base will produce a solution of
Similarly, titrating a weak acid with a strong base will produce a solution of