How is atomic size measured?
1 Answer
Two methods are X-ray (or electron or neutron) diffraction and microwave spectroscopy.
Explanation:
Atomic size is the distance from the nucleus to the edge of the electron cloud.
The edge of the electron cloud is not well defined, so chemists use other definitions of atomic size, and they all give slightly different numbers.
Metallic radius
One way is to assume that the radius of an atom is half the distance between adjacent atoms in a solid.

This technique is best suited to metals, in which the atoms are arranged in specific patterns. The results are often called metallic radii.
A beam of X-rays passes through the crystal and creates a pattern of clear spots in a characteristic pattern.

Scientists can work "backwards" from this pattern and calculate the crystal structure and the metallic radii.
Covalent radius
The covalent radius is one-half the distance between the nuclei in a homonuclear diatomic molecule.

The bond lengths are measured by X-ray, electron, and neutron diffraction and also by microwave spectroscopy.
The rotational motions of molecules are quantized, and the transitions between energy levels occur in the microwave region.
The rotational spectrum for
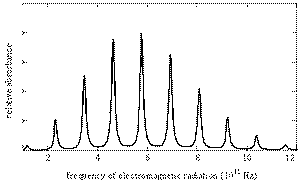
(from chemwiki.ucdavis.edu)
Chemists can use the spectra to calculate the moment of inertia of the molecule and hence the
They repeat the measurements for many different molecules and then calculate the "best fit" value for the covalent radius of each atom.

