What happens to acetic acid at high temperature?
2 Answers
Acetic acid is a distillable liquid. Its boiling point is
Explanation:
At higher temperature, the acid of course will boil.
If you heat acetic acid to very high temperatures, it can dehydrate into acetic anhydride.
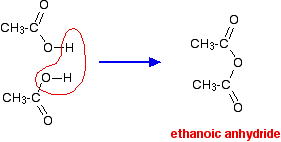
But it works even better with an acid catalyst.
Unfortunately my book doesn't show me what the mechanism is, but it's probably like this:
I recall doing a lab with this reaction in high school, and we went from carboxylic acids like butyric acid (which smells like vomit) to more tame-smelling anhydrides (cherry, banana, etc).
Granted, there are better ways to make anhydrides (since heating to high temperatures can be difficult to accomplish depending on the equipment you have), like using acyl chlorides with sodium acetate, but anyways...


