Why is benzene carcinogenic?
1 Answer
Sometimes benzene, but polycyclic aromatic hydrocarbons, more likely, can oxidize in the body to form epoxides, some of which are susceptible to a carcinogenic pathway.
Benzene rings in general can oxidize in the body with the "help" of the enzyme cytochrome P450.
A component in DNA, called 2'-deoxyguanosine, has an
Once that happens, the structure of the 2'-deoxyguanosine no longer matches the pocket on the DNA double helix, and basically, that leads to improper gene transcription that can lead to cancerous mutations (Organic Chemistry, Bruice, pg. 652).
Furthermore, there are two reaction pathways the arene oxide can take: rearrangement into a phenol, or simply getting attacked by a nucleophile like 2'-deoxyguanosine.
If the rate of rearrangement into a harmless phenol is too slow, then the arene oxide is likely a carcinogen.
Two examples of carcinogenic arene oxides are 4,5-benzo[a]pyrene oxide and 7,8-benzo[a]pyrene oxide, and you can find precursors of those in "cigarette smoke, automobile exhaust, and charcoal-broiled meats" as benzo[a]pyrene (Organic Chemistry, Bruice, pg. 653).
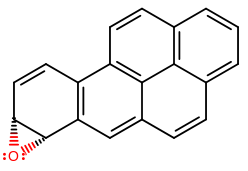
(7,8-benzo[a]pyrene oxide)
Those don't rearrange fast enough, and so they are more carcinogenic than smaller polycyclic aromatic hydrocarbons.

