What is the conjugate base of #CH_3NH_3^+#?
1 Answer
Methylamine.
Explanation:
As you know, a Bronsted - Lowry acid is a chemical species that donates a proton,
The species that accepts that proton acts as a Bronsted - Lowry base.
Now, a conjugate base is a chemical species that can reform a Bronsted - Lowry acid by accepting a proton. In essence, you can go from an acid to its conjugate base by removing a proton, and from the conjugate base to the original acid by adding a proton.
In your case, you're starting from an acid, the methylammonium ion,
The conjugate base of the methylammonium ion will have one less proton. Keep in mind that a proton carries a
#"CH"_3"NH"_3^(color(red)(+)) -> "CH"_3"NH"_2 + color(red)("H"^(+))#
The conjugate base of the methylammonium ion is
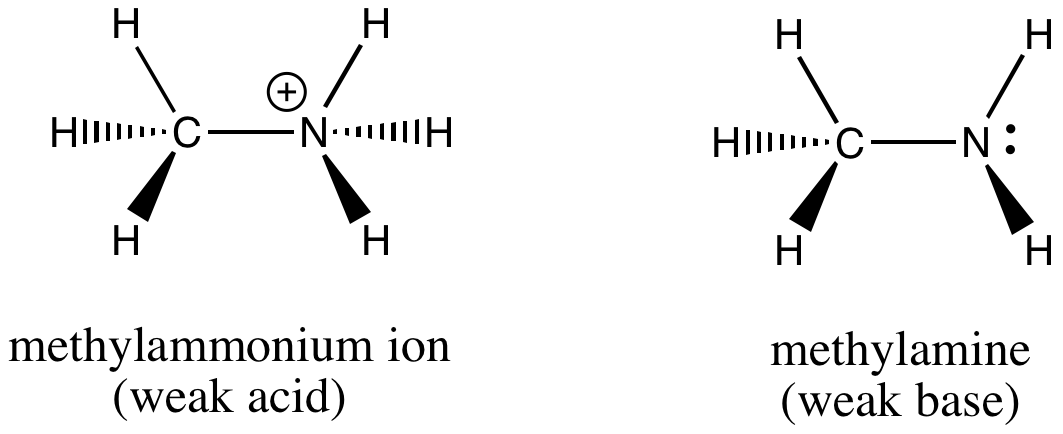
Notice that you can reform the methylammonium ion by adding a proton to its conjugate base.
In aqueous solution, the methylammonium ion acts as a weak acid. The water molecule acts as bases.
#overbrace("CH"_3"NH"_text(3(aq])^(+))^(color(green)("acid")) + overbrace("H"_2"O"_text((l]))^(color(purple)("base")) rightleftharpoons overbrace("CH"_3"NH"_text(2(aq]))^(color(green)("conjugate base")) + overbrace("H"_3"O"_text((aq])^(+))^(color(purple)("conjugate acid"))#

