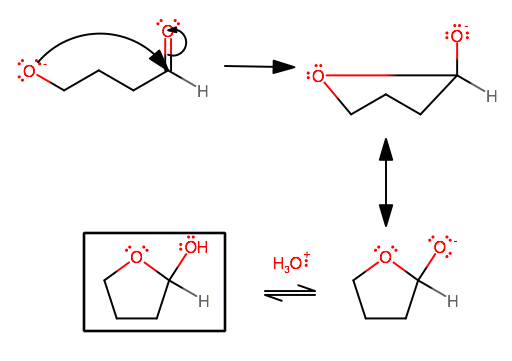
What is the major difference between a cyclic hemiacetal and a cyclic acetal?
1 Answer
A cyclic acetal is a protecting group. It forms when you react an aldehyde in acid with an organic diol like ethanediol. There are two nucleophilic attacks on the same carbonyl carbon here, one of which is an intramolecular ring formation.
A cyclic hemiacetal is formed when you protonate an aldehyde that has a hydroxyl group far enough away that an intramolecular ring formation can be made, but with only one nucleophilic attack on the same carbonyl carbon, not two.
Or, instead of protonating the aldehyde's carbonyl oxygen, you could somehow deprotonate the hydroxyl cation (like by making a sodium derivative of the compound), which is what is shown at the bottom.
CYCLIC ACETAL MECHANISM
- We're starting with an already-protonated acetone. It was protonated with some hydronium. The pKa of ethanediol is about
#14.2# , compared to that of water, which is about#15.7# , so water better retains its protonated (hydronium) form, meaning water is a slightly better "proton-transferring agent", so to speak, than ethanediol. - Proton transfer part 1. We are trying to remove the top
#"OH"# group since we want to replace it with the protecting group. - Proton transfer part 2. This is the step that helps that occur, by turning the top hydroxide into a better leaving group.
- Tetrahedral collapse makes the
#"OH"_2^(+)# leave, giving another protonated intermediate. This is susceptible to intramolecular ring formation. - And it happens. The ring formation.
- Regenerate the dominant form of the acid catalyst.
CYCLIC HEMIACETAL MECHANISM
We're kinda going to skip straight to the nucleophilic attack because you get the idea by now.