What is the total angular momentum quantum number?
1 Answer
DISCLAIMER: This can be a tough topic, so ask questions if you need to.
ATOMIC TERM SYMBOLS
We should see this in the context of atomic term symbols, which describe:
- The type of orbital (
#s# ,#p# , etc) - The number of unpaired electrons
- The possibility for spin-orbit coupling
An atomic term symbol looks like this:
#\mathbf(""^(2S + 1)L_J)# where:
#S# is the total spin angular momentum of all#m_s# for each individual electron in the set of orbitals; it's a fast way of telling you how many unpaired electrons there are.#2S + 1# is called the spin multiplicity, which basically is a more concise way of telling you what#S# tells you, and gives rise to the terminology "singlet state", "doublet state", etc. It's a formal thing.#L# is similar to#l# , which is the orbital angular momentum, i.e. the shape of the orbital.#J# is the total angular momentum, which is just a value that collapses#S# and#L# into another variable.
P1 CONFIGURATION
So, let's take an example. Let's say we had a
It's the simplest example that isn't too simple:
DETERMINING TOTAL SPIN ANGULAR MOMENTUM
To determine
You should get:
#color(green)(S) = +["1/2"] = color(green)(+"1/2")#
Determine the spin multiplicity, and you should get:
#color(green)(2S + 1) = color(green)(2)#
DETERMINING ORBITAL ANGULAR MOMENTUM
Now, since it's a
(Had there been two or more electrons,
DETERMINING TOTAL ANGULAR MOMENTUM
Finally,
#\mathbf(J = L + S, L + S - 1, . . . , |L - S|)#
Here, we have:
#J = 1 + "1/2", 1 + "1/2" - 1, . . . , |1 - "1/2"|#
but
#color(green)(J = "1/2", "3/2")#
OVERALL ATOMIC TERM SYMBOLS
So, we can write out the term symbols as:
#""^(2S + 1)L_J#
#""^(2S + 1)L_(L pm S)#
#-> color(blue)(""^2 P_"1/2", ""^2 P_"3/2")#
WHAT DOES IT MEAN?
From this, we can work backwards and make the following interpretations:
- The number of unpaired electrons is
#1# , because#2S + 1 = 2# , so#S = "1/2"# . - Because
#S = "1/2"# ,#J - S = L = "3/2" - "1/2"# , so#L = 1# , and we are looking at a#p# orbital. - We do NOT know whether there are
#1# or#5# electrons total in the three#2p# orbitals because either configuration gives one unpaired electron. But we do know that there are either#1# or#5# , so the possible electron configurations are#p^1# and#p^5# . - We know that in an energy level diagram, we should see two energy states:
#""^2 P_"1/2"# and#""^2 P_"3/2"# , which are very close together. Because of an effect called spin-orbit coupling, the two energy levels, which would otherwise be the same, split slightly in a magnetic field (sometimes giving differences of less than#"1 nm"# in the wavelength).
As an example of why this can be important, it tells you that there are two different
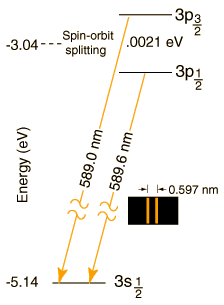
Both give a yellow emission line upon relaxation, but there are two transitions, not one.

