What are the similarities and differences between diffusion and osmosis?
1 Answer
Explanation:
Diffusion is very interesting; it happens in our everyday life. When you fart in a classroom, everyone can smell it. When we fart, the gas diffuses in the air(because in the air there is less concentration of your fart-gas) and that's why it can be smelled in your whole classroom.
Remember that our nature always has a way of balancing things; it always desires equilibrium.
When an area of lower concentration(air in the classroom) encounters a medium with high concentration(your fart), it spreads in the air until equilibrium is met.
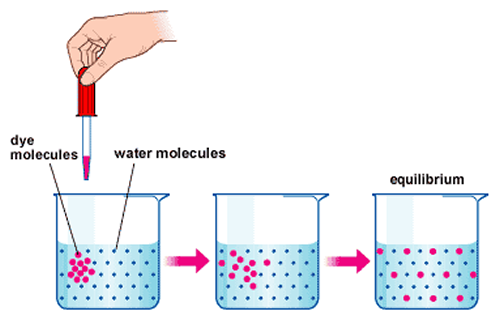 http://astrocampschool.org/wp-content/uploads/2015/12/DiffusionDiagrampng
http://astrocampschool.org/wp-content/uploads/2015/12/DiffusionDiagrampng
The same is true when we place a dye in water; the dye spreads all over the water until a uniform color is met; equilibrium is met. This is diffusion.
Osmosis is however, the diffusion of water happening when a semi-permeable membrane is in between them.
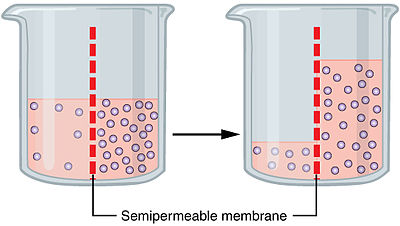 https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/0307_Osmosisjpg/400px-0307_Osmosisjpg
https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/0307_Osmosisjpg/400px-0307_Osmosisjpg
Because it is semi-permeable, it allows water to go in and out but not other substances. And therefore, to reach equilibrium, water goes in to dilute. Water can also go out leading to a more concentrated solution, based on what we call tonicity, which is the relative concentration of solutions(solution 1, Higher concentration. Solution 2, lower concentration) that determine the direction and extent of diffusion
