Question #c1499
1 Answer
Here's what's going on here.
Explanation:
In order to understand why a solution of calcium chloride,
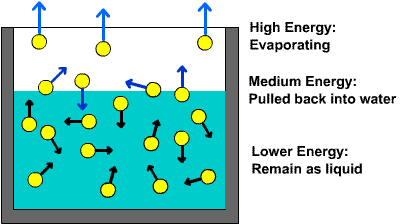
As you know, a liquid is said to boil when its vapor pressure becomes equal to the atmospheric pressure.
When you start heating a liquid, you are essentially increasing the kinetic energy of its molecules. As temperature increases, more and more molecules located at the surface of the liquid will have enough energy to overcome the intermolecular force of attraction and escape into the gaseous state.
By comparison, you freeze a liquid by taking away heat, which is equivalent to decreasing the kinetic energy of its molecules.
As temperature decreases, the intermolecualr forces of attraction will overpower the kinetic energy of the molecules
Now, when you dissolve a solute in pure water, the molecules of solute will affect the boiling point and the freezing point of the liquid.
The attraction between the molecules of solute and the molecule of solvent will reduce the number of molecules of solvent that are near the surface of the liquid.
Moreover, some molecules of solute will be close to the surface of the liquid, which essentially blocks solvent molecules from being able to escape into gaseous state.
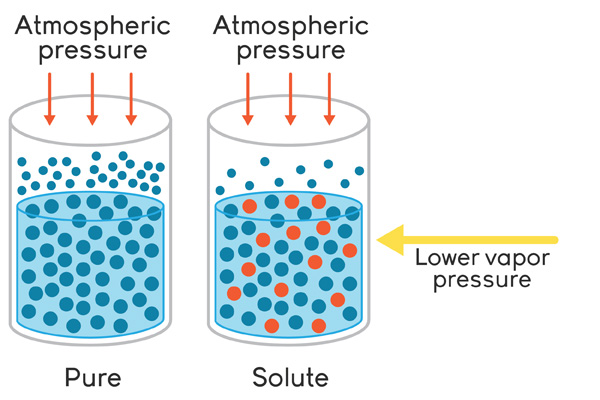
This means that in order to make the liquid boil, you need to provide it with more heat to get the vapor pressure of the solution to match the atmospheric pressure
This attraction between the molecules of solute and the molecules of solvent will disrupt the freezing of the liquid as well.
You will have to decrease the temperature more in order to allow for the intermolecular forces of attraction to pin the molecules into place
So, when you dissolve calcium chloride in water, you will get complete dissociation of the solid
#"CaCl"_text(2(aq]) -> "Ca"_text((aq])^(2+) + 2"Cl"_text((aq])^(-)#
Notice that the solution contains three moles of particles, one mole of calcium cations and two moles of chloride anions, for every one mole of calcium chloride dissolved!
These ions will interact with the water molecules and essentially increase the solution's boiling point and decrease its freezing point.
Boiling-point elevation and freezing-point depression are colligative properties because they depend on the number of particles produced in solution, not on the nature of those particles.
This is why, for example, electrolytes like calcium chloride increase the boiling point of the solution more than non-electrolytes.
Non-electrolytes dissolve without dissociation. One mole of sugar will get you one mole of sugar particles in solution.
The van't Hoff factor,
For calcium chloride, the van't Hoff factor will be equal to
#"1 mol CaCl"_2" dissolved" -> "3 moles ions produced in solution"#