Question #0b448
1 Answer
Here's what's going on here.
Explanation:
In order to be able to understand what's going on when the solution is cooled from
Notice that the solubility curve shows that at
What that means is that at that temperature, you can only dissolve
A saturated solution is a solution in which you have an equilibrium between the solid solute and particles of solute that are being dissolved into solution.
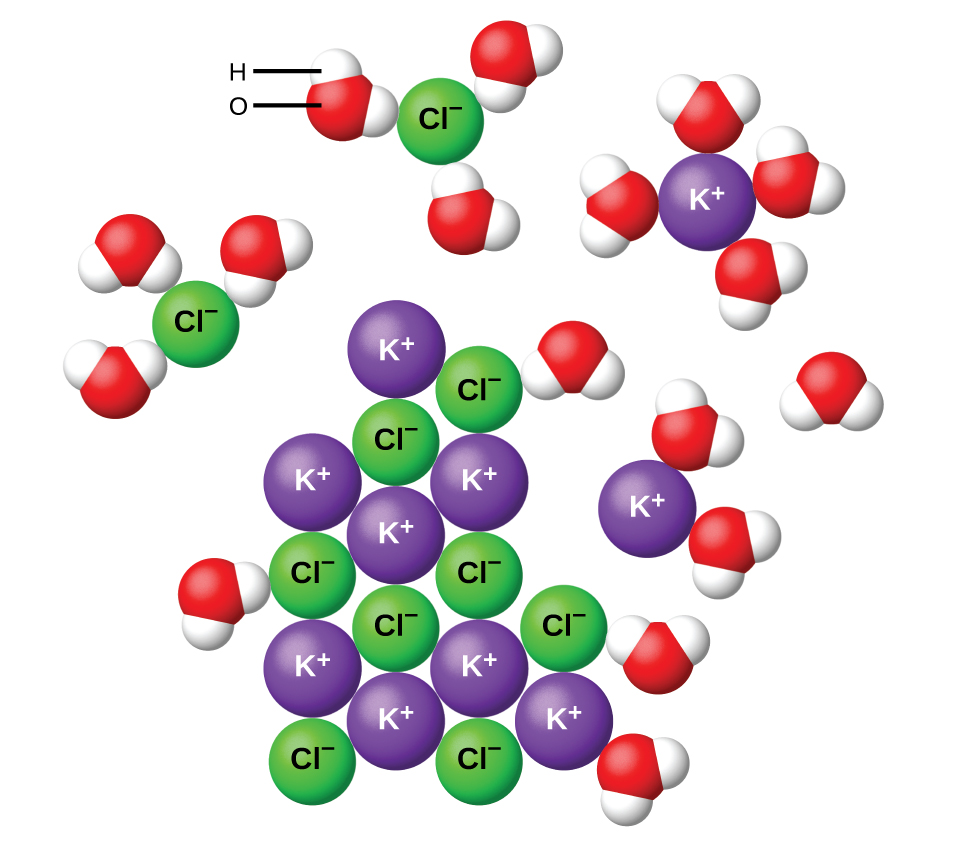
At
If you add more than
Now, notice that the solubility of potassium chloride changes with temperature. As temperature decreases from
This means that the solution will hold increasingly smaller amounts of potassium chloride.
As temperature decreases, the average kinetic energy of the water molecules decreases as well. This implies that the water molecules will be less effective at overcoming the ionic bonds that are holding the solute particles together, and thus less effective at breaking apart the solid's crystal structure.
Moreover, decreasing the temperature of the solution also stabilizes the solid's crystal structure because the vibration of the solid's particles will decrease.
As a result, fewer potassium cations,
At
This tells you that the difference between the amount of potassium chloride that could be dissolved at
In simple terms, the solvated ions will once again become part of the crystal structure. Dissolved potassium chloride will become solid potassium chloride. For these values, you will have
#m_"precipitate" = "42 g" - "28 g" = "14 g KCl" -># will precipitate
#m_"dissolved" = "28 g KCl" -># will remain dissolved
Sometimes if you cool the solution slow enough, you will get a supersaturated solution, which is a solution that can hold more dissolved solute than the solubility limit at that given temperature.

