Question #73ba2
1 Answer
Two atoms share electron pairs.
Explanation:
You are correct, a covalent bond is indeed formed when two atoms share electrons.
Although this is an oversimplification, the difference between covalent bonding and ionic bonding can be explained by the position of the bonding electrons.
When two atoms form a covalent bond, they share the bonding electrons. In simple terms, the electrons they share will spend equal amounts of time surrounding both atoms.
When two atoms form an ionic bond, they do not share the bonding electrons. What happens here is that one atom will take both electrons and develop an overall negative charge
The other atom will lose the electrons it contributed to the bond and develop an overall positive charge
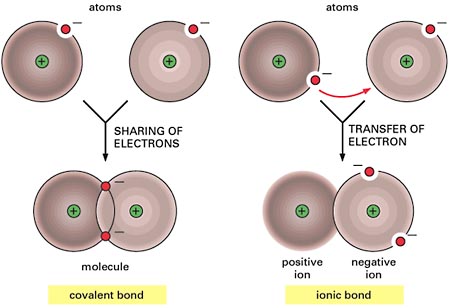
In your case, three out of the four options actually describe an ionic bond. Options
Option
Since opposite charges attract, the electrostatic force of attraction will then kick into high gear and pull these ions together

