How do i determine which bond will be the most polar without being given the electronegativitiy numbers of each atom? Also, how do I determine which bond will be the longest?
Im working on a worksheet to prepare for my upcoming exam, and the questions are: For the listen molecule, which bond will be the most polar?
For the listed molecule, which bond will be the longest?
Ill attempt to give the lewis dot:
F H
Cl-C-C-O
H H H
Edit: The format of the lewis dot got messed up when posting and I dont know how to fix it. The first C should have F on top and H below. The 2nd C should have H on top and bottom. And the O should have H on the bottom.
The fluorine and 3 hydrogens are all bonded to their corresponding C's by a single bond,and the oxygen and hydrogen are also bonded by a single bond.
The answer to the polarity question is C-F. However I answered C-H because I remember learning that the farther apart the atoms are, the more polar they are (greater difference in electronegativities). So why is the C-F bond more polar than the C-H?
The answer to the length is C-C. This one makes no sense to me. I answered once again C-H.
Could someone please give me clarification on this?
Im working on a worksheet to prepare for my upcoming exam, and the questions are: For the listen molecule, which bond will be the most polar?
For the listed molecule, which bond will be the longest?
Ill attempt to give the lewis dot:
F H
Cl-C-C-O
H H H
Edit: The format of the lewis dot got messed up when posting and I dont know how to fix it. The first C should have F on top and H below. The 2nd C should have H on top and bottom. And the O should have H on the bottom.
The fluorine and 3 hydrogens are all bonded to their corresponding C's by a single bond,and the oxygen and hydrogen are also bonded by a single bond.
The answer to the polarity question is C-F. However I answered C-H because I remember learning that the farther apart the atoms are, the more polar they are (greater difference in electronegativities). So why is the C-F bond more polar than the C-H?
The answer to the length is C-C. This one makes no sense to me. I answered once again C-H.
Could someone please give me clarification on this?
1 Answer
To know this you pretty much do have to kind of memorize a few electronegativities. I don't recall ever getting a table of electronegativities on an exam.
From the structure, you have:
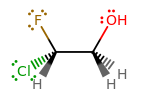
I remember the following electronegativities most because they are fairly patterned:
#"EN"_"H" = 2.1# #"EN"_"C" = 2.5# #"EN"_"N" = 3.0# #"EN"_"O" = 3.5# #"EN"_"F" = 4.0# #"EN"_"Cl" = 3.5#
Notice how carbon through fluorine go in increments of
#Delta"EN"_("C"-"Cl") = 1.0# #Delta"EN"_("C"-"H") = 0.4# #Delta"EN"_("C"-"C") = 0.0# #Delta"EN"_("C"-"O") = 1.0# #Delta"EN"_("O"-"H") = 1.4#
So naturally, with the greatest electronegativity difference of
When the electron distribution is polarized and drawn towards a more electronegative atom, the less electronegative atom has to move inwards because its nucleus was previously favorably attracted to the electrons from the other atom.
That means generally, the greater the electronegativity difference between two atoms is, the shorter you can expect the bond to be, insofar as the electronegative atom is the same size as another comparable electronegative atom.
However, examining actual data, we would see that on average, in conditions without other bond polarizations occuring:
#r_("C"-"Cl") ~~ "177 pm"# #r_("C"-"C") ~~ "154 pm"# #r_("C"-"O") ~~ "143 pm"# #r_("C"-"F") ~~ "135 pm"# #r_("C"-"H") ~~ "109 pm"# #r_("O"-"H") ~~ "96 pm"#
So it is not necessarily the least electronegativity difference that gives the longest bond.
Therefore, you cannot simply consider electronegativity. Examining the radii of the atoms, you should notice that chlorine is the biggest atom in the compound.
#r_("Cl") ~~ "79 pm"# #r_("C") ~~ "70 pm"# #r_("H") ~~ "53 pm"# #r_("O") ~~ "60 pm"#
So assuming the answer is truly
- The
#"C"-"F"# bond polarization makes the carbon more electropositive (which is true). - The now more electropositive carbon wishes to attract bonding pairs from chlorine closer, thereby shortening the
#"C"-"Cl"# bond, and potentially the#"C"-"H"# bond (which is probably true). - The shortening of the
#"C"-"Cl"# bond is somehow enough to be shorter than the#"C"-"C"# bond (this is debatable).

