Does this mean that SO2 grabs an Oxygen out of every second OH(-) group present in the solution?
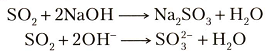
1 Answer
Feb 10, 2016
Probably not. It means that the sulfur oxides are the acid anhydrides of common acids.
Explanation:
So what is an acid anhydride? It is the acid LESS the elements of water,

