How do you find the mass of an atom?
1 Answer
Feb 19, 2016
One way is to use a mass spectrometer.
You might think we could find the atomic mass of an atom by adding up the masses of its protons, neutrons, and electrons.
Let's use the numbers to calculate the atomic mass of carbon-12 (which is defined as 12.000 u).
The masses are:
- proton = 1.0073 u
- neutron = 1.0087 u
- electron = 0.000 55 u
The atom should have a mass of 12.099 u by adding the individual masses, but its actual mass is only 12.000 u.
This mass defect arises because energy is released when the particles come together, and this energy equals the mass defect (
Mass Spectrometry
One way to determine the mass of an atom is to use a mass spectrometer.
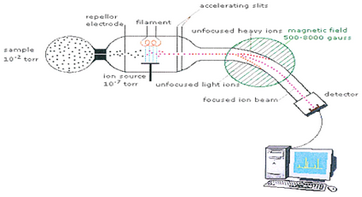
(from www.pharmatutor.org)
In this technique,
- A small sample ionized by a beam of electrons.
- The ions are sorted and separated according to their mass and charge by passing them through a magnetic field.
- The separated ions are detected and the results are fed to a computer, which calculates the masses.

