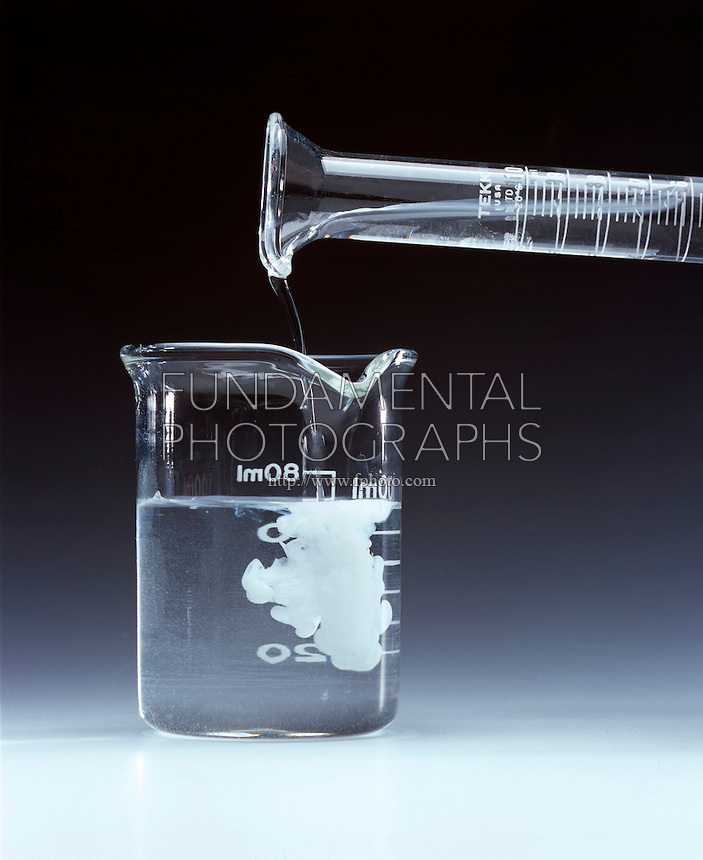
Why doesn't barium nitrate react with sulfuric acid?
1 Answer
It does react with sulfuric acid!
Explanation:
Barium nitrate,
Barium sulfate is insoluble in water and will precipitate out of solution.
The balanced chemical equation for this double replacement reaction looks like this
#"Ba"("NO"_3)_text(2(aq]) + "H"_2"SO"_text(4(aq]) -> "BaSO"_text(4(s]) darr + 2"HNO"_text(3(aq])#
The complete ionic equation for this reaction looks like this
#"Ba"_text((aq])^(2+) + 2"NO"_text(3(aq])^(-) + 2"H"_text((aq])^(+) + "SO"_text(4(aq])^(2-) -> "BaSO"_text(4(s]) darr + 2"H"_text((aq])^(+) + 2"NO"_text(3(aq])^(-)#
To get the net ionic equation, eliminate spectator ions, i.e. the ions that are present on both sides of the equation
#"Ba"_text((aq])^(2+) + color(red)(cancel(color(black)(2"NO"_text(3(aq])^(-)))) + color(red)(cancel(color(black)(2"H"_text((aq])^(+)))) + "SO"_text(4(aq])^(2-) -> "BaSO"_text(4(s]) darr + color(red)(cancel(color(black)(2"H"_text((aq])^(+)))) + color(red)(cancel(color(black)(2"NO"_text(3(aq])^(-))))#
This will give you
#"Ba"_text((aq])^(2+) + "SO"_text(4(aq])^(2-) -> "BaSO"_text(4(s]) darr#
Barium sulftate is a white insoluble solid that precipitates out of solution.