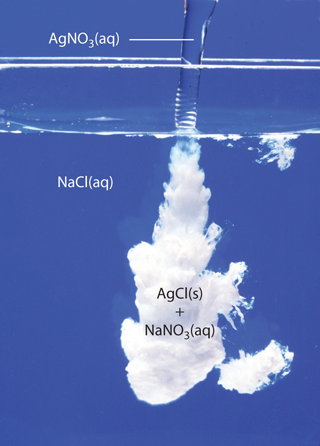
You're essentially dealing with four ionic compounds that are insoluble in aqueous solution.
Silver chloride, "AgCl"AgCl, is a white precipitate that can be obtained by mixing a solution of a soluble ionic compound that contains the silver(I) cation, "Ag"^(+)Ag+, with a solution of a soluble ionic compound that contains the chloride anion, "Cl"^(-)Cl−.
Lead(II) chromate, "PbCrO"_4PbCrO4, is a yellow precipitate that can be obtained by mixing a solution of a soluble ionic compound that contains the lead(II) cation, "Pb"^(2+)Pb2+, with a solution of a soluble ionic compound that contains the chromate anion, "CrO"_4^(2-)CrO2−4.

Copper(II) sulfide, "CuS"CuS, is a black precipitate that can be formed by mixing a solution of a soluble ionic compound that contains the copper(II) cation, "Cu"^(2+)Cu2+, with a solution of a soluble ionic compound that contains the sulfide anion, "S"^(2-)S2−.

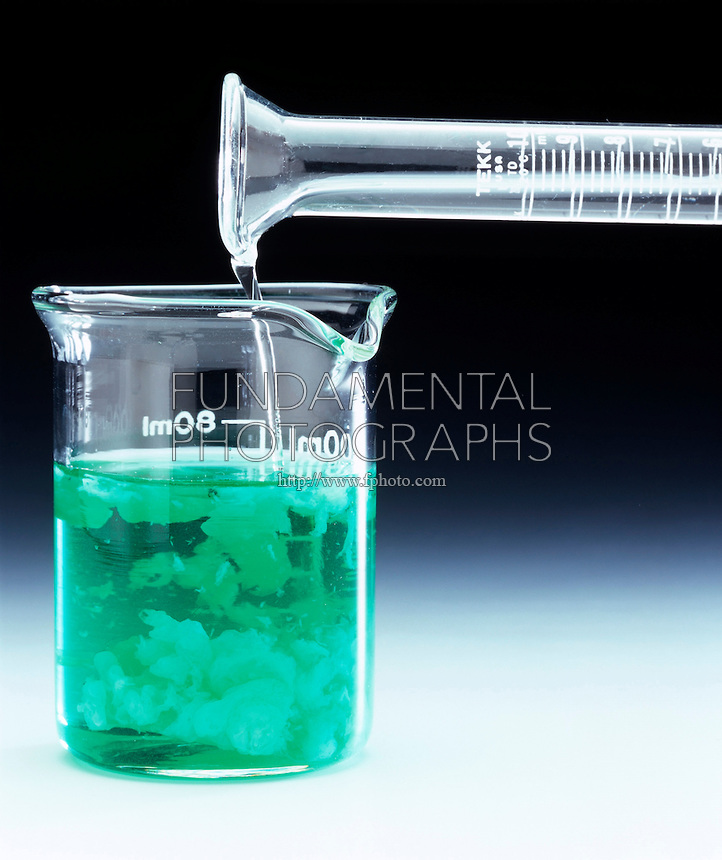
Finally, nickel(II) hydroxide, "Ni"("OH")_2Ni(OH)2, is a green precipitate that results when a solution of a soluble ionic compound that contains the nickel(II) cation, "Ni"^(2+)Ni2+, is mixed with a solution of a soluble ionic compound that contains the hydroxide anion, "OH"^(-)OH−.