Question #14ae4
1 Answer
Mar 20, 2016
There are two main ways to calculate an element's valency.
Explanation:
The valency is the number of electrons in an element's outer shell and can be found in two ways.
- With a Periodic Table
The Periodic Table is organized in part by valence electrons. As seen in the table below, along the top of the Periodic Table are numbers (0-7). These correspond to valency. The noble gases (far right) have 8 electrons in their valence shell and, as the shell is full, have a valency of 0. The alkali metals (far left) have one electron in their outer shell and a valency of 1, shown by the 1 above it. Valency can therefore be easily determined by a Periodic Table for all elements except transition metals.
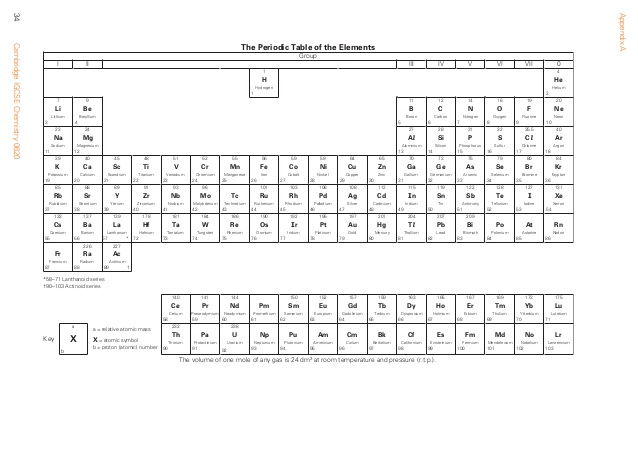
- Without a Periodic Table
The valency can also be found by using an element's total electron number. Since there are two electrons in the first shell and eight in each following shell, the valency can easily be calculated. For instance, carbon has six electrons. Two go into the first shell, leaving four for the second shell. Carbon has a valency of four because it has four electrons in its outer shell.

