Why do we see different wavelengths of light as different colors?
1 Answer
This question can be answer from different perspectives, e.g. biological, philosophical, physico-chemical, but in generic terms, the wavelengths implies in different energy amount.
Explanation:
This question can be answer from different perspectives, e.g. biological, philosophical, physico-chemical, but in generic terms, they wavelengths implies in different energy contents.
Biologically speaking
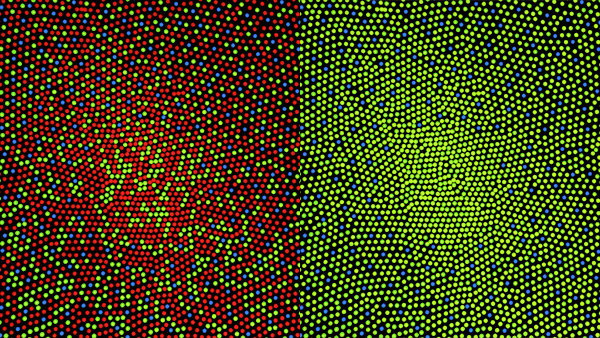
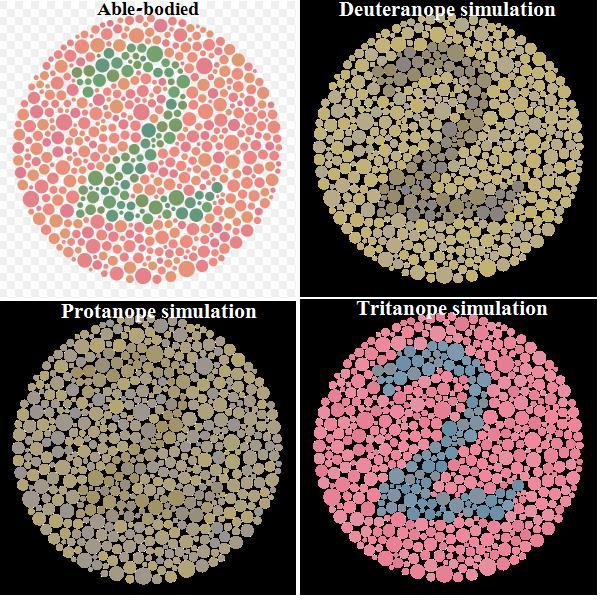
Our eyes, more precisely the retina, is composed of different cell-light sensitive. There are three different types, RGB, red green and blue respectively, all the other colors are "secondary." Daltonism is the problem to see red due to lack of red-cell sensitive.
Physically speaking
Energy content, wavelength size, goes from radio to gamma rays, being radio in meters and gamma in nanometers. Therefore, different color is associated to different energy intensity, being reddish the color of "low energy", ultraviolet of "high energy", as so they are used to clean devices in hospitals.
Doing the math
We see different wavelengths of light as different colors because they are associated to different wavelength, which activates different cells in the retina. You can try to answer the question from another perspective, such as why a red surface is red, but that is another story.

