When calculating the mass of a Uranium-235 nucleus, can we just subtract the mass of the electrons from the given mass of a Uranium-235 atom?
I am confused as to whether the mass equivalent of the binding energy of the electrons needed to be taken into account in the calculation of the mass of the uranium-235 nucleus as the electrons are "removed" from the atom.
I am confused as to whether the mass equivalent of the binding energy of the electrons needed to be taken into account in the calculation of the mass of the uranium-235 nucleus as the electrons are "removed" from the atom.
1 Answer
Yes.
The electrostatic binding energy of electrons is a small quantity in comparison to the nuclear mass and therefore, can be ignored.
Explanation:
We know if we compare the combined mass all the nucleons with sum of individual masses of all these nucleons, we will find that
the combined mass is less than sum of individual masses.
This is known as mass defect or sometimes also called mass excess.
It represents the energy that was released when the nucleus was formed, called binding energy of nucleus.
Let's assess the binding energy of electrons to the nucleus.
Take the example of Argon for which ionization potentials are given for its 18 electrons here.
Argon atom has 18 protons and therefore it has charge of
Actual ionization energy for removing all 92 electrons of Uranium-235 needs to be calculated by taking sum of ionization energy of each electrons. Now we know that all electrons are probability-wise located farther from the nucleus. However with increase of nuclear charge size of inner orbitals becomes small.
To make an assessment we use a multiplying factor
Right Hand side of the approximation
We know that
and also 1 a.m.u. with the help of
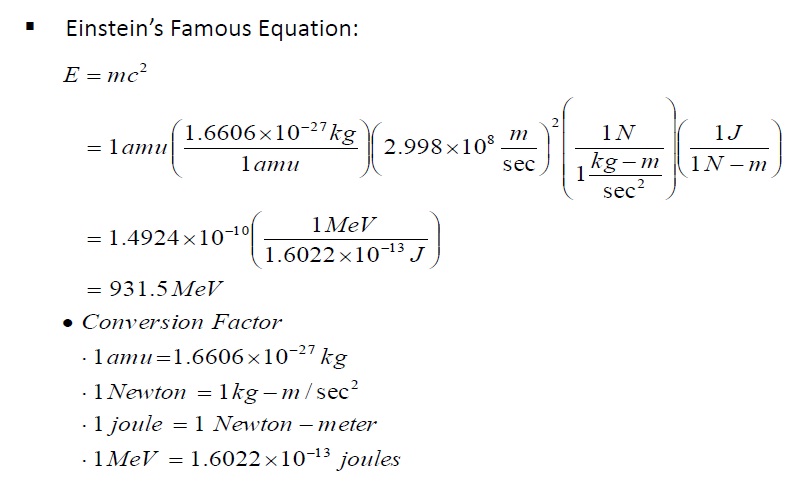
As such assessed electrostatic binding energy of 92 electrons to uranium nucleus is around
This is a very small quantity even as compared to mass of smallest nucleus and therefore can be ignored for all practical purposes.
