Question #06929
1 Answer
Any substance that can deliver calcium cations or sulfate anions to the solution.
Explanation:
Calcium sulfate,
#"CaSO"_ (4(s)) rightleftharpoons "Ca"_ ((aq))^(2+) + "SO"_(4(aq))^(2-)#
Calcium sulfate's solubility product constant,
Since
Now, in order to decrease the solubility of calcium sulfate, you must essentially find away to push the equilibrium even further to the left.
As you know, equilibrium reactions are governed by Le Chatelier's Principle, which states placing a stress on a system at equilibrium will cause that equilibrium to shift in such a way as to reduce that stress.
In your case, you can place a stress on the position of the equilibrium by increasing the concentration of either calcium cations,
This will cause the equilibrium to shift to the left because the reverse reaction consumes the extra ions by reforming some of the solid.
So, take a look at the Solubility Rules for ionic compounds
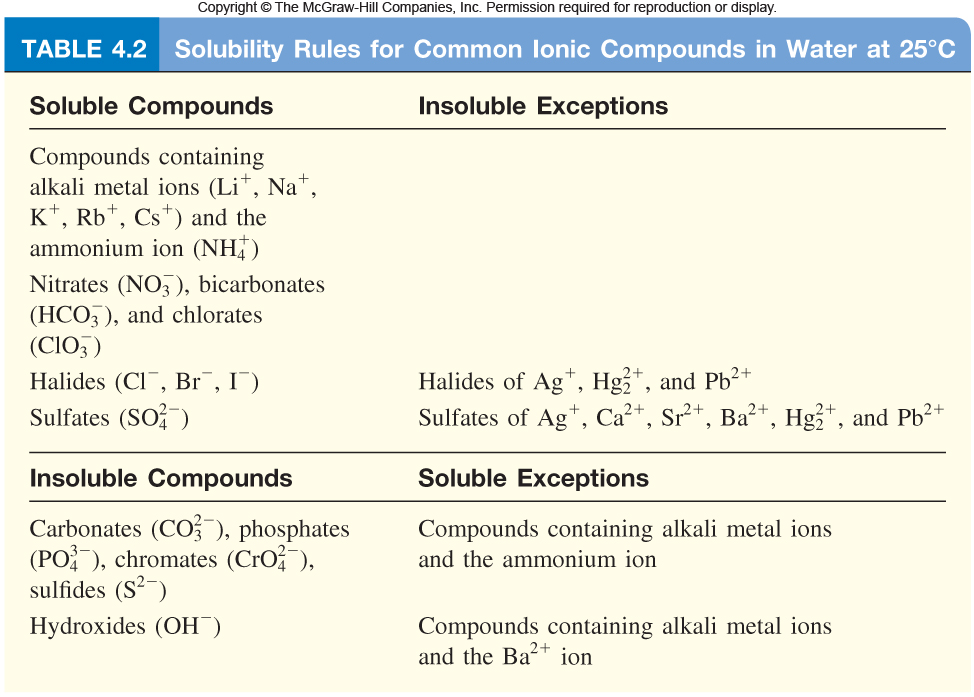
Your goal here is to find substances that can dissolve in aqueous solution to produce calcium cations or sulfate anions.
Let's start with calcium cations. Notice that all the compounds that contain the nitrate anion,
This means that if you were to add calcium nitrate,
#"Ca"("NO"_ 3)_ (2(aq)) -> "Ca"_ ((aq))^(2+) + 2"NO"_(3(aq))^(-)#
Now do the same for the sulfate anion. Notice that you are given a list of cations that form insoluble compounds when paired with the sulfate anion. Pick one that is not on that list.
A good choice would be sodium sulfate,
#"Na"_ 2"SO"_ (4(aq)) -> 2"Na"_ ((aq))^(+) + "SO"_(4(aq))^(2-)#
Dissolving sodium sulfate in a saturated solution of calcium sulfate will decrease the latter's solubility because of the increase in the concentration of sulfate anions.

