Question #0d6d1
1 Answer
Here's how that would work.
Explanation:
The idea is that when you dissolve an ionic compound that is considered insoluble in aqueous solution, an equilibrium is established between the dissolved ions and the undissolved solid.
The common ion effect refers to the decrease or increase in the solubility of such an ionic compound that results from providing ions that affect the position of the aforementioned equilibrium.
In your case, the following equilibrium will be established when lead(II) chloride is placed in aqueous solution
#"PbCl"_ (2(s)) rightleftharpoons "Pb"_ ((aq))^(2+) + 2"Cl"_((aq))^(-)#
Now, think Le Chatelier's Principle here. In order to affect the position of this equilibrium, you must place a stress on it. As you know, the system will respond by shifting in such a way as to reduce* that stress.
You're interested in increasing the solubility of lead(II) chloride, which means that you want the forward reaction to be favored. This tells you that you must make the equilibrium shift to the right.
To do that, you must decrease the concentration of lead(II)cations,
As a response, the position of the equilibrium will shift to the right, dissolving more solid to compensate for the decrease in the concentration of dissolved ions.
Now, take a look at the Solubility Rules for ionic compounds in aqueous solution
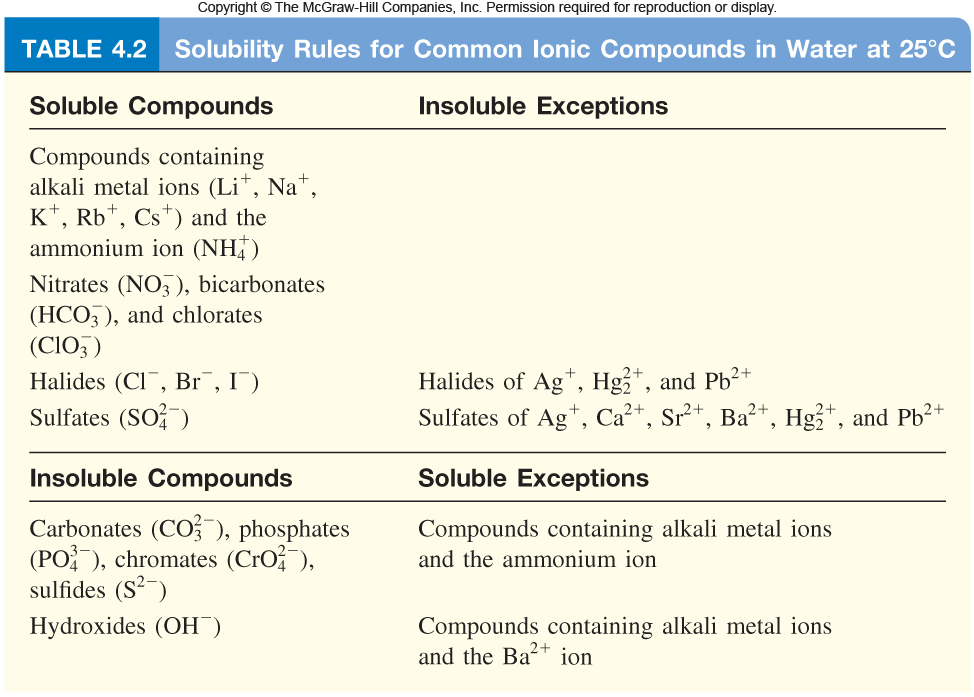
Your goal here is to find two substances that will remove the two ions from solution. In other words, you must find two soluble ionic compounds that will deliver ions that can precipitate either lead(II) cations or chloride anions to the solution.
Let's start with the lead(II) cations. Notice that lead(II) cations also form an insoluble solid with the sulfate anions,
One example of such a salt would be ammonium sulfate,
#("NH"_ 4)_ 2"SO"_ (4(aq)) -> 2"NH"_ (4(aq))^(+) + "SO"_(4(aq))^(2-)#
The sulfate anions will then combine with the lead(II) cations to form lead(II) sulfate,
#"Pb"_ ((aq))^(2+) + "SO"_ (4(aq))^(2-) -> "PbSO"_(4(s)) darr#
Now for the chloride anions. Notice that silver cations,
#"AgNO"_ (3(aq)) -> "Ag"_ ((aq))^(+) + "NO"_(3(aq))^(-)#
These cations will then combine with the chloride anions to form silver chloride,
#"Ag"_ ((aq))^(+) + "Cl"_ ((aq))^(-) -> "AgCl"_((s)) darr#
And there you have it, two substances that can increase the solubility of lead(II) chloride in aqueous solution.

